Drug scheduling refers to the process of determining the classification of a health product. Health Canada and the National Association of Pharmacy Regulatory Authorities (NAPRA) have distinct roles in the drug scheduling process in Canada, with each organization performing specific functions. The process begins with Health Canada, who determines whether the drug requires a prescription for sale. NAPRA’s scheduling process starts when a drug is classified as non-prescription by Health Canada and the result of NAPRA’s process determines the drug’s conditions for sale in Ontario.
Below we explore three areas of drug scheduling:
Scheduling “by reference”
NAPRA’s National Drug Schedules are referenced in O. Reg. 264/16, s.3 under the Drug and Pharmacies Regulation Act. Therefore, whenever NAPRA makes a scheduling decision or change, it is automatically in force for Ontario pharmacies. To keep up to date on NAPRA’s scheduling decisions or changes, registrants can follow National Drug Schedules (NDS) Updates.
Prescriptions Drugs: Schedule I
Health Canada classifies a drug as requiring a prescription for sale by placing it on the Prescription Drug List in accordance with the Food and Drug Regulations, Part C under Canada’s Food and Drugs Act.
Drugs listed in the schedules to the Controlled Drugs and Substances Act, including narcotics, controlled drugs, benzodiazepines and targeted substances are not on the Prescription Drug List, however prescription status is conferred by their respective regulations.
As not all prescription drugs are captured in the National Drug Schedules, registrants should access the Prescription Drug List and the schedules to the Controlled Drugs and Substances Act, or Health Canada’s Drug Product Database to verify whether a drug requires a prescription. A drug with prescription status at the federal level requires a prescription in all provinces and territories. These drugs are in NAPRA Schedule I and not reviewed by NAPRA.
Non-prescription Drugs: Schedules II, III and U
When a newly marketed drug is given non-prescription (over-the-counter or OTC) status, or a drug is removed from the Prescription Drug List by Health Canada, the drugs conditions of sale are determined by the provinces and territories. NAPRA’s National Drug Scheduling Advisory Committee (NDSAC) will review the scheduling of these drugs only when a submission from a pharmaceutical manufacturer is made to NAPRA. If the manufacturer does not make a submission to the NDSAC, the drug’s schedule will not be reviewed, and the drug will remain in Schedule I as per NAPRA’s policy.
The specific process that must be followed during each drug scheduling review is outlined in NAPRA’s By-law No. 2 and Rules of Procedures. Since the review process embodies a “cascading principle” to assess drugs against specific scheduling factors, it is possible (although rare) for a non-prescription status drug to be placed in Schedule I.
If a submission for review is received, the NDSAC reviews the submission and makes an interim recommendation for scheduling followed by a 30-day consultation period. Once NAPRA’s Executive Committee makes a final recommendation for scheduling, the National Drug Schedules is updated and is in force in Ontario.
As of 2024, all natural health products approved for sale under the federal Natural Health Product Regulations (i.e., products with a Natural Product Number (NPN) or Drug Identification Number-Homeopathic Medicine (DIN-HM) from Health Canada) will be considered outside the scope of NAPRA’s National Drug Schedules as explained in the Background on Update to NAPRA NHP Policy. Similarly, products that do not meet the definition of “drug” in the Drug and Pharmacies Regulation Act (DPRA) are not subject to the DPRA or National Drug Schedules and their sale is not restricted to a pharmacy.
Key Takeaways
A drug given prescription status by Health Canada at the federal level always requires a prescription in Ontario. For drugs given non-prescription status by Health Canada, Ontario regulations require pharmacies to follow the NAPRA National Drug Schedules. Registrants should know how to verify a drug’s schedule and associated conditions for sale by referencing the appropriate resources.Test Your Knowledge
Use the below educational tool to anonymously test your knowledge of drug scheduling in Ontario
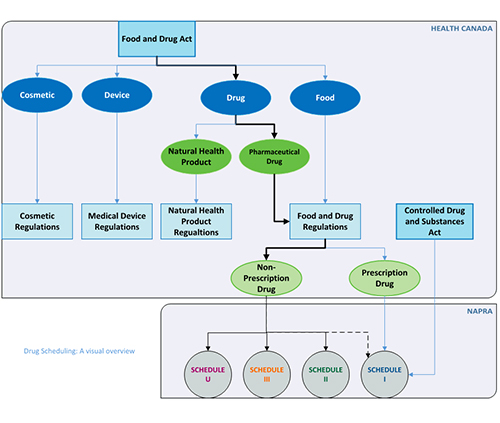
DRUG SCHEDULING – A VISUAL OVERVIEW