Ian Stewart, B.Sc.Phm., R.Ph.
During the dispensing process, the Drug Identification Number (DIN) and the tablet or capsule appearance are two factors commonly used to confirm that the correct drug is being dispensed.
However, pharmacists and pharmacy technicians must be aware of the potential for error when the medication is dispensed in the original sealed stock bottle.
CASE:
A patient requested a refill of his Janumet® 50mg/500mg tablets. The total quantity to be dispensed was 180 tablets.
As the manufacturer’s stock bottle contain a childproof lid, the pharmacy assistant preparing the tablets to be dispensed decided to use the original stock bottle and not transfer the tablets into a prescription vial. As a result, two sealed bottles of Janumet® 50mg/500mg (60 tablets each) and in error, one sealed bottle of Janumet® 50mg/1000mg (60 tablets) were selected, labelled and provided to the pharmacist for checking.
The prescription label obscured most of the information on the stock bottle. Hence, upon verifying the product being dispensed, the pharmacist failed to identify the dispensing error.
As a result, the patient received one bottle of the incorrect tablets.
The patient selected and consumed the incorrect strength of Janumet® for approximately one week before contacting the pharmacy to enquire about the change in tablet appearance.
Fortunately, no ill effects were experienced.
POSSIBLE CONTRIBUTING FACTORS:
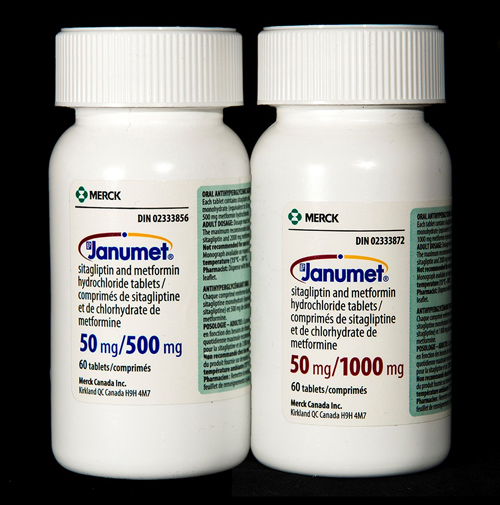
- The bottles of both strengths of Janumet® tablets are similar in size, shape, and appearance (see photo).
- Both strengths of Janumet® were stored next to each other enhancing the potential for selecting the incorrect product.
- The actual tablets could not be seen by the dispensing pharmacist as all bottles were sealed.
- The pharmacy uses bar code technology to confirm that the correct drug is being dispensed. In this instance, the pharmacy assistant scanned one bottle of Janumet® 50mg/500mg three times instead of scanning each bottle being dispensed.
- The pharmacy assistant’s placement of the prescription label on the original stock bottles obscured most of the identifying information except the Drug Identification Number. However, the first six digits of the DIN for both products are identical. Hence, a quick glance at the DINs may fail to identify the difference.
RECOMMENDATIONS:
- Develop a system to separate the various strengths of look-alike products. Options include dividers, baskets or separate shelves.
- Use extra care when dispensing products in the original sealed package where the appearance of the actual tablet or capsule cannot be seen. This is especially critical when the product packaging is similar in size and shape.
- Always examine and scan each product being dispensed. Never scan the same product multiple times.
- If the product must be dispensed in the original package, the pharmacy assistant should not obscure any information on the label before it is checked by the pharmacist.
- The prescription label may be initially attached to the stock bottle with the use of a rubber band. The pharmacist may then attach the prescription label to the product after it is confirmed to be correct.
- Patients and caregivers should be encouraged to ask questions, especially if something does not seem right.
Please continue to send reports of medication errors in confidence to Ian Stewart at: ian.stewart2@rogers.com. Sharing your experience can prevent similar occurrences at other practice sites. Please ensure that all identifying information (e.g. patient name, pharmacy name, healthcare provider name, etc.) are removed before submitting.
as part of the AIMS program, pharmacists and pharmacy technicians must:
- Anonymously record all medication incidents and near misses via the AIMS medication event reporting platform.
- Document appropriate details of medication incidents and near misses in a timely manner to support accuracy.
- Analyze the incident in a timely manner for causal factors and commit to taking appropriate steps to minimize the likelihood of recurrence of the incident.
- Promptly communicate the appropriate details of a medication incident or near miss, including causal factors and actions taken as a result, to all staff.
PUTTING THE AIMS PROGRAM INTO PRACTICE USING THIS INCIDENT EXAMPLE
Imagine you are the pharmacist who answers the phone when the patient calls to inquire about why their Janumet looks different than previous refills.
After remedying the situation and reassuring the patient you will look into what could have caused the mix up, you are ready to record the incident on the AIMS Pharmapod platform.
ANALYZE, DOCUMENT & RECORD
After entering some demographic information on the patient and prescriber, the next step is entering the incident details.
QUICK TIP: The information you will need in this first step is the patient’s date of birth, gender and prescription number in addition to the prescriber’s license number and name (optional fields).Using the drop-down menu labeled “What happened? (Incident Type)”, you identify the incident type as “incorrect concentration or strength” and describe briefly what happened from your perspective in the free-form comment section.
QUICK TIP: If you were not directly involved with the incident, you may choose to discuss with staff members who were directly involved to gain a more thorough understanding of the incident.The next step is thinking about and recording the factors that may have contributed to the incident.
The AIMS platform can support multiple contributing factors per incident.
In this particular case, you could select the following contributing factors in the recording platform:
- Environmental, staffing, or workflow problem
Rationale:
- The bottles of both strengths of Janumet® tablets are similar in size, shape, and appearance (see photo).
- Both strengths of Janumet® were stored next to each other enhancing the potential for selecting the incorrect product
- Lack of quality control or independent check system
Rationale:
- The actual tablets could not be seen by the dispensing pharmacist as all bottles were sealed.
- The bar code of one bottle of Janumet® 50mg/500mg was scanned three times instead of each bottle being dispensed.
- The placement of the prescription label on the original stock bottles obscured most of the identifying information except the Drug Identification Number.
- The first six digits of the DIN for both products are identical. Hence, a quick glance at the DINs may fail to identify the difference.
Finally, in the incident details section, the form also includes selections for the stage of the dispensing process in which the incident occurred – in this case the primary stage is “product selection” and the secondary stage “dispensing/preparation.”
The remaining sections of the incident reporting form include details of the medication involved and the staff involved.
QUICK TIP: At any time your work can be saved as a draft to facilitate form completion in busy environments.SHARE LEARNINGS & FOLLOW UP
Thinking through the incident using the structured format of the incident recording form allows you to gain a better understanding of what happened, why it happened and where in the dispensing process it happened. This information allows for the development of impactful solutions that, once implemented, would reduce or eliminate the recurrence of similar incidents in the future. The recommendations above are examples of solutions that can be implemented in any pharmacy to prevent similar incidents. The process of thinking about medication incidents and near misses in this way promotes continuous quality improvement in your pharmacy’s work processes leading to improved patient safety and reduced patient harm. Recording incidents and near misses in AIMS also contributes towards shared learnings throughout the province and broader system improvements.