Grant Fuller, PharmD Candidate1,2
Certina Ho, RPh, BScPhm, MISt, MEd, PhD 2,3
1 School of Pharmacy, University of Waterloo
2 Institute for Safe Medication Practices Canada
3 Leslie Dan Faculty of Pharmacy, University of Toronto
CASE EXAMPLE
Mr. Jones, a patient of your pharmacy, states that he has been feeling nauseated and has had a few episodes of diarrhea ever since he picked up his new prescription two days ago. Upon examining his pharmacy profile and the medication vial he has brought in, it becomes apparent that he has received another patient’s metformin prescription by accident, instead of his intended medication, atorvastatin.
INTRODUCTION
Every patient has the right to be informed about their healthcare. This includes the right to be promptly notified when an incident associated with medication therapy has occurred. In fulfilling this commitment to their patients, as well as meeting their ethical and organizational obligations, practitioners need to understand when disclosure is appropriate or necessary and how to properly disclose medication incidents.
In addition to disclosure, there can be other challenges in responding to medication incidents. The Assurance and Improvement in Medication Safety (AIMS) Program relies on practitioners to share details of medication incidents when they are discovered. However, this requires an environment in which staff feel comfortable reporting medication incidents without undue consequences to their self-image, status, or career.1 (For further information, refer to a previous article published in the Summer 2018 edition of Pharmacy Connection: Safe Pharmacies Need Psychological Safety.)
Very often, practitioners involved in medication incidents experience psychological and physical consequences which include extreme sadness, difficulty sleeping, and intrusive memories.2 Recognizing these responses and the need to support practitioners are often overlooked in the aftermath of a medication incident. (For further information, refer to a previous article published in the Spring 2019 edition of Pharmacy Connection: Aftermath of a Medication Incident: Caring for the Patient, the Family, but also the Healthcare Professional.)
To support practitioners, we have provided a framework for medication incident disclosure and applied it to the case example above. This framework has been adapted from the Canadian Patient Safety Institute (CPSI) Canadian Disclosure Guidelines and a previously-published continuing education lesson for pharmacy technicians on How to Handle a Medication Error.3,4 We also consulted the Canadian Medical Protective Association (CMPA) article, Disclosing harm from healthcare delivery: Open and honest communication with patients.5 We would like to refer readers to these original resources for further information.
SUGGESTED DISCLOSURE FRAMEWORK
Immediate Actions3,4
After a medication incident is discovered, there are immediate actions that must be taken before beginning the disclosure process:
- Attend to the affected patient(s); ensure their care needs are met.
- Take immediate measures to prevent similar safety risks from harming other patients or staff.
Is Disclosure of the Medication Incident Needed? 3,4
After any immediate safety concerns are addressed, practitioners must decide whether disclosing the medication incident to the patient is appropriate or necessary.
- Consider the degree of harm the patient experienced or could have reasonably experienced as a result of the incident (Figure 1)
- “Harm” refers to incidents that reached the patient and resulted in temporary or permanent impairment (including mental, physical, sensory functions and pain) in body functions or structures6
- “No Harm” refers to incidents that reached the patient but resulted in no injury6
- “Near Miss” refers to events that could have resulted in patient harm but did not reach the patient6
- When in doubt about disclosing a medication incident, consider whether a person would reasonably want to know about the incident.
Figure 1 – Circumstances When Disclosure is Appropriate or Necessary3
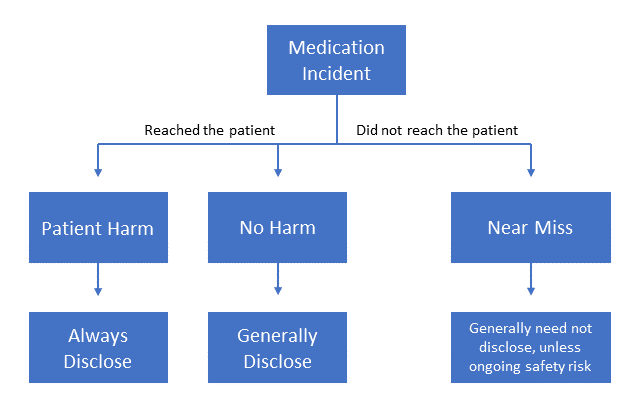
Source: Canadian Patient Safety Institute. Canadian disclosure guidelines: being open and honest with patients and families; November 2011.
Apologies3,4
Apologies are crucial to the disclosure process and should be offered as they make patients feel validated and respected. Legislation exists in several provinces, including Ontario, to protect practitioners from legal liability due to apologizing.7 Features of an effective approach are presented below:
- Communicate genuine sincerity about the medication incident.
- Use a personal tone including terms such as “I” or “We.”
- Use appropriate non-verbal gestures (body language, tone of voice, facial expressions).
- Assure that harm did not result from anything the patient or family did or did not do.
Preparing the Disclosure3,4
After it has been determined that a disclosure is needed, consider the following when preparing for the initial meeting:
- Schedule an in-person disclosure meeting at the earliest practical opportunity. Select a time that is convenient for the patient and family and a place that is private and free of interruptions. Allow adequate time for a complete discussion about the incident.
- The most responsible healthcare provider who is involved should facilitate the disclosure. All others who played a role in the incident should be prepared to discuss relevant events with the patient and family.
- Anticipate emotions; both the patient and practitioners should have supports available at the disclosure meeting if needed.
- Assign a staff member as the primary contact for the patient and family throughout this process.
Disclosure3,4
The initial disclosure is a crucial step as it provides an opportunity for the patient and family to understand what the medication incident was and why/how it might have happened.
- Focus on the events that led to the medication incident, using clear and understandable terminology. Avoid speculation and assigning blame.
- Encourage the patient and family to discuss the incident from their point of view.
- Discuss any changes to the ongoing care of the patient in consultation with the patient’s primary healthcare provider.
- Keep a record of the discussion. Allow the patient and family to review the documentation to ensure everyone agrees on the facts.
Continued Feedback3,4
The disclosure process requires continued dialogue with the patient and family rather than a single discussion. After the initial disclosure meeting and when the medication incident has been fully reviewed and analyzed:
- Communicate new findings about the incident to the patient and family members.
- Reinforce, update, or correct information provided in previous meetings.
- Discuss any improvements or changes made to prevent similar events from occurring.
- Provide continued practical and emotional support to the patient and family.
CASE EXAMPLE – APPLICATION
Immediate Actions3,4
Ensure that Mr. Jones is not experiencing any symptoms of potentially dangerous hypoglycemia (e.g. confusion, dizziness, vision changes) due to the inadvertent administration of metformin. Follow up on the management of his nausea and diarrhea in consultation with his primary healthcare provider. Secure the metformin prescription and provide Mr. Jones with his correct medication, atorvastatin. Ensure a new metformin prescription was prepared for the intended patient.
Is Disclosure of the Medication Incident Needed? 3,4
Mr. Jones has experienced “harm” in the form of nausea and a few episodes of diarrhea due to this incident. Disclosure is necessary as it helps Mr. Jones understand that a medication incident occurred and what circumstances might have contributed to this event.
Apologies3,4
A suggested initial apology can be: “I am very sorry about this, Mr. Jones. It appears you received the wrong medication. I want to emphasize that none of this is your fault and we will work with you to figure out why/how this happened.“
Preparing the Disclosure3,4
The pharmacy should make every effort to schedule an in-person disclosure meeting with Mr. Jones as soon as possible. The Designated Manager (DM) or pharmacy manager will facilitate communication with Mr. Jones and oversee the disclosure process as the DM is the most responsible healthcare provider involved in the patient’s care in this case. The staff member who gave the medication to Mr. Jones at pick-up should also be prepared to discuss the incident with him.
A possible arrangement would be: The disclosure meeting is scheduled next Sunday when the pharmacy is closed to allow for privacy and the time needed for a thorough discussion. Mr. Jones is advised that he may bring his family for support if desired.
Disclosure3,4
Here is a potential disclosure: “Mr. Jones, I want to take some time to discuss the events that have led up to you receiving the wrong medication. When you picked up the prescription, we neglected to ask you for an additional patient identifier or information besides your name, such as your address or date of birth. You share a very similar name to one of our other patients. These factors contributed to you receiving the wrong prescription. I have contacted your family physician who agrees that no changes to your current care are needed. Again, we are very sorry about what happened. Would you like to share your thoughts on this incident with us?“
Continued Feedback3,4
While reviewing and analyzing the medication incident, the pharmacy team discovered that Mr. Jones had refused counselling when he picked up his medication as he was in a rush. As a result of this incident, the pharmacy has changed its processes related to prescription pick-up. All staff will request secondary patient identifiers upon prescription pick-up and counselling will be given for all prescriptions (new prescriptions and refills). These process improvements and workflow changes will be shared with Mr. Jones along with any new findings the pharmacy discovers.
CONCLUSION
When a medication incident occurs, the ensuing process – from reporting and disclosure to the point where eventual system improvements are implemented – can be complicated. Having both a framework and the knowledge to appropriately disclose medication incidents are important, however, they represent just one step in addressing the challenges of medication incident reporting and learning. Improving how we deal with medication incidents will take continued effort on the part of organizations, teams and individual practitioners.
REFERENCES
- Edmondson AC, Lei Z. Psychological safety: The history, renaissance, and future of an interpersonal construct. Annu Rev Organ Psychol Organ Behav 2014; 1: 23-43.
- Burlison JD, Quillivan RR, Scott SD, Johnson S, Hoffman JM. The Effects of the Second Victim Phenomenon on Work-Related Outcomes: Connecting Self-Reported Caregiver Distress to Turnover Intentions and Absenteeism. Journal of Patient Safety 2016 Nov. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5413437/pdf/nihms-788857.pdf
- Disclosure Working Group. Canadian disclosure guidelines: being open and honest with patients and families. Edmonton, AB: Canadian Patient Safety Institute; 2011. Available from: https://www.patientsafetyinstitute.ca/en/toolsResources/disclosure/Pages/default.aspx
- Ho C, Kawano A. How to handle a medication error. TECH talk CE 2013;May:4-10. Available from: http://www.canadianhealthcarenetwork.ca/files/2013/05/TechTalkMay-2013.pdf
- Canadian Medical Protective Association. Disclosing harm from healthcare delivery: Open and honest communication with patients. 2017. Available from: https://www.cmpa-acpm.ca/en/advice-publications/browse-articles/2015/disclosing-harm-from-healthcare-delivery-open-and-honest-communication-with-patients
- ISMP Canada. Definitions of Terms. Available from: https://www.ismp-canada.org/definitions.htm
- Government of Ontario. Apology Act. 2009. Available from: https://www.ontario.ca/laws/statute/09a03













