Adrian Boucher, BSc, PharmD Student 1,2
Sonya Dhanjal, BSc, PharmD Student 2,3
Jim H. Kong, RPh, BSc, PharmD 2
Certina Ho, RPh, BScPhm, MISt, MEd, PhD 1,2,3
1 Leslie Dan Faculty of Pharmacy, University of Toronto
2 Institute for Safe Medication Practices Canada
3 School of Pharmacy, University of Waterloo
BACKGROUND
Although pharmacy professionals and pharmacy organizations aim to provide error-free patient care, medication incidents are inevitable. Medication incidents are defined as any preventable events that may cause inappropriate medication use or patient harm while the medication is in the control of the healthcare professional or patient; these events occur when vulnerable medication-use systems and/or human factors affect prescribing, transcribing, dispensing, administration, and monitoring practices. 1,2
Community pharmacies in Canada dispense over 600 million prescriptions annually, 3 but only a fraction of medication incidents will reach the patient and an even smaller proportion will result in harm. However, these incidents are associated with significant costs to patients and the healthcare system. In particular, they may lead to negative business implications for community pharmacies as a result of direct legal and financial costs, tarnished reputations, and decreased customer loyalty. On the other hand, these incidents often reveal broader system flaws, and thus, represent excellent opportunities for incident analysis and shared learning.
In an effort to identify and address factors that lead to harmful medication incidents, pharmacy organizations have developed and implemented incident reporting systems. At the local level, reporting systems are frequently an integral part of continuous quality improvement (CQI) programs, and as such, are associated with long-term improvements in organizational learning and patient safety culture. 4 At the national level, reporting systems provide representative data for large-scale aggregate analysis, enabling healthcare stakeholders to better understand contributing factors that may have led to medication incidents, and aiding practitioners, pharmacies, and regulatory authorities in developing and sharing strategies to prevent recurrence. A multi-incident analysis is one form of aggregate analysis that is used to qualitatively analyze reported incident data to extract contributing factors and develop safety measures to prevent the incidents from re-occurring. By organizing and reviewing narrative incident data with common themes based on composition or origin, a multi-incident analysis can offer system-based learning that cannot be obtained through other analysis methodology.
The Institute for Safe Medication Practices Canada (ISMP Canada) established a national incident data repository for community pharmacies through its community pharmacy incident reporting (CPhIR) program. This article explores a multi-incident analysis conducted on harm-related medication incidents reported to CPhIR. The following sections contain an overview of the reported medication incidents and results from the analysis. Specific examples of reported incidents are also provided for reflection and to aid in developing strategies that can be customized to any practice setting. By systematically examining such incidents, root causes can be identified and process changes can be made to reduce the likelihood of similar errors from occurring again.
METHODS
A total of 971 medication incidents associated with patient harm were extracted from the ISMP Canada Community Pharmacy Incident Reporting (CPhIR) Program (http://www.cphir.ca) from 2009 to 2017. Sixty-two incidents were excluded due to insufficient narrative incident description for analysis. A total of 909 incidents were included for the multi-incident analysis, which was conducted by two independent ISMP Canada analysts. Themes, sub-themes, contributing factors, and recommendations to address patient safety gaps corresponding to harm-related incidents were then derived from this analysis.
RESULTS
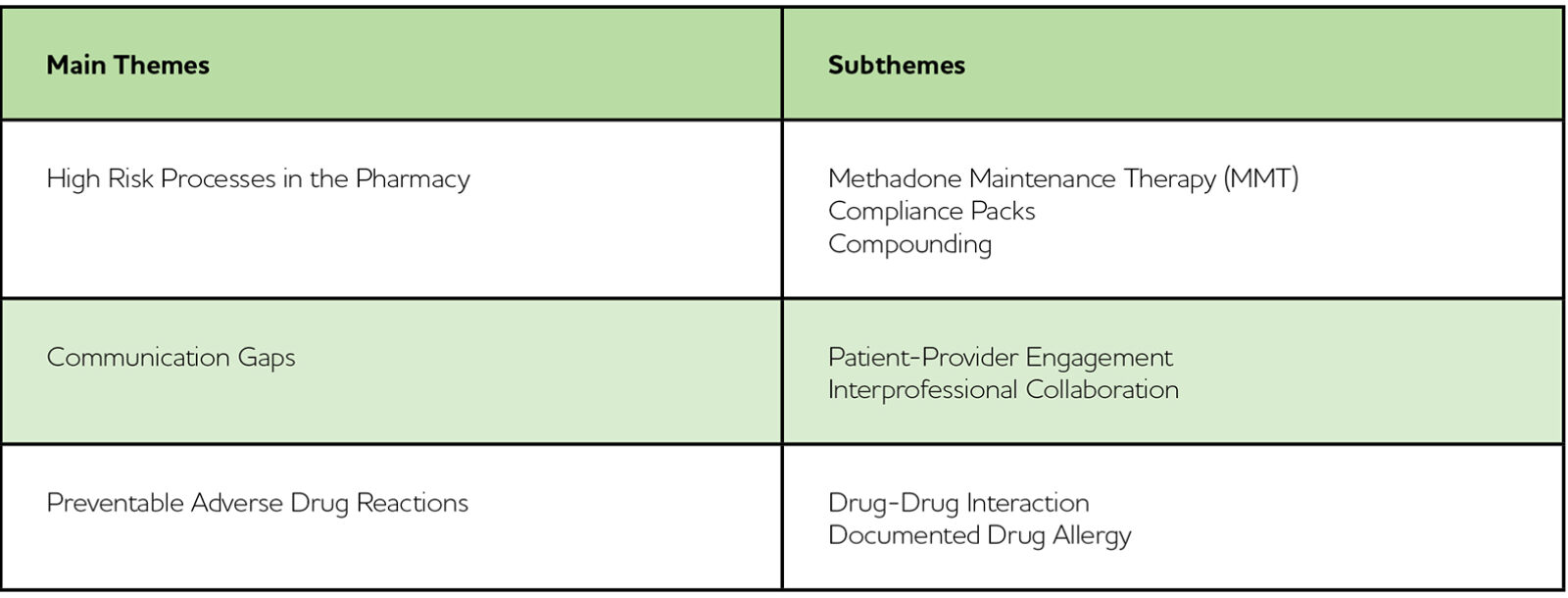
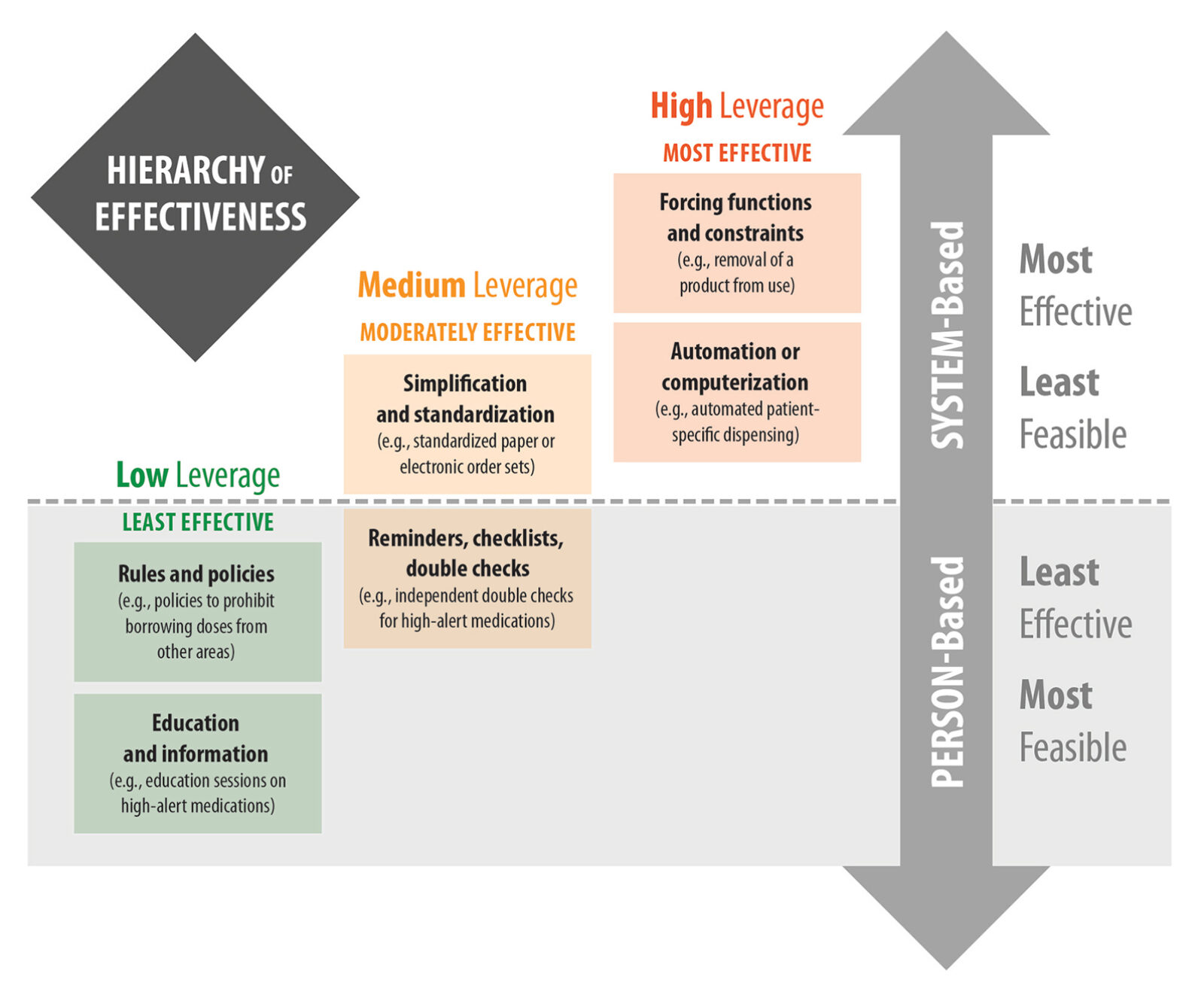
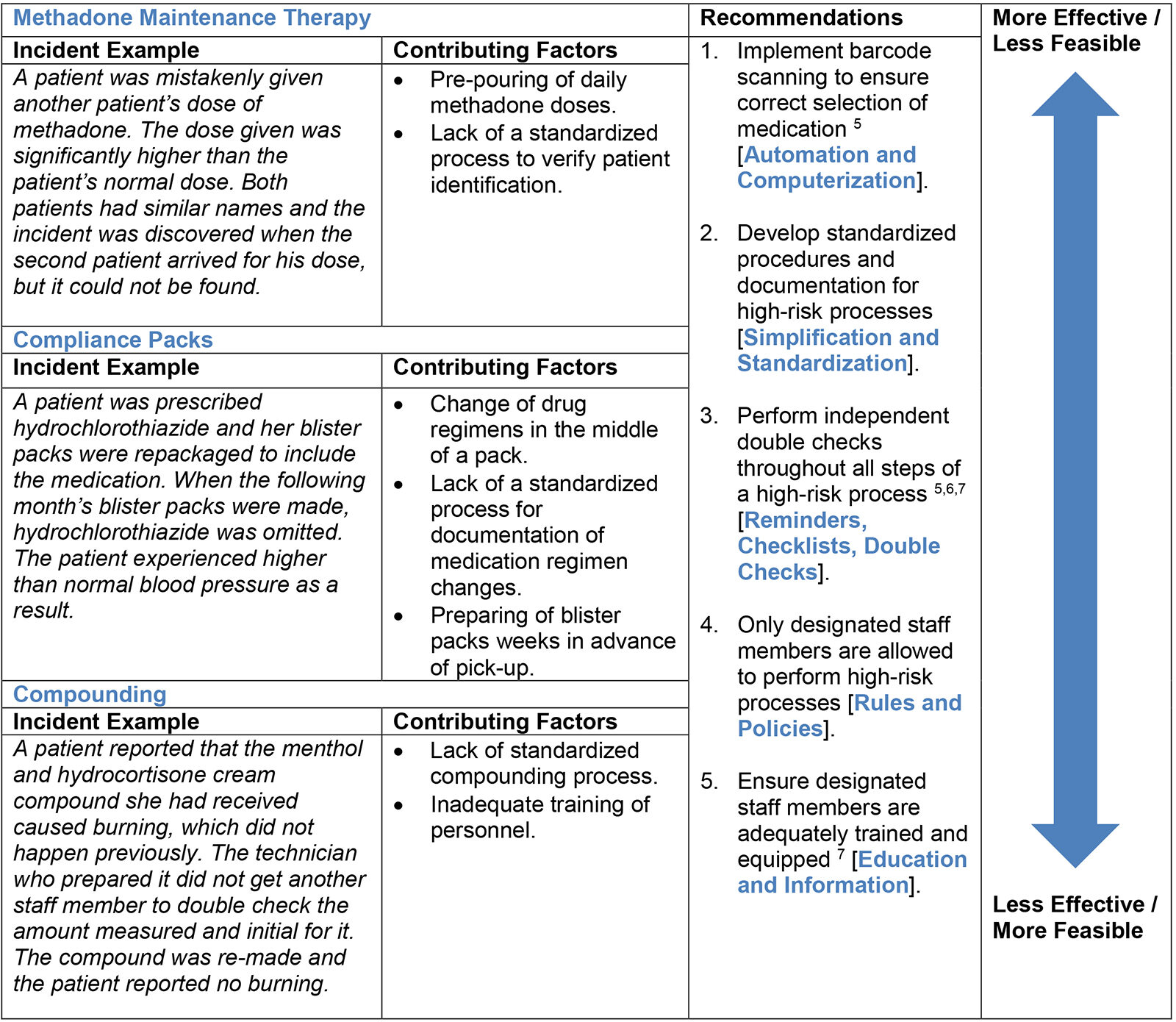
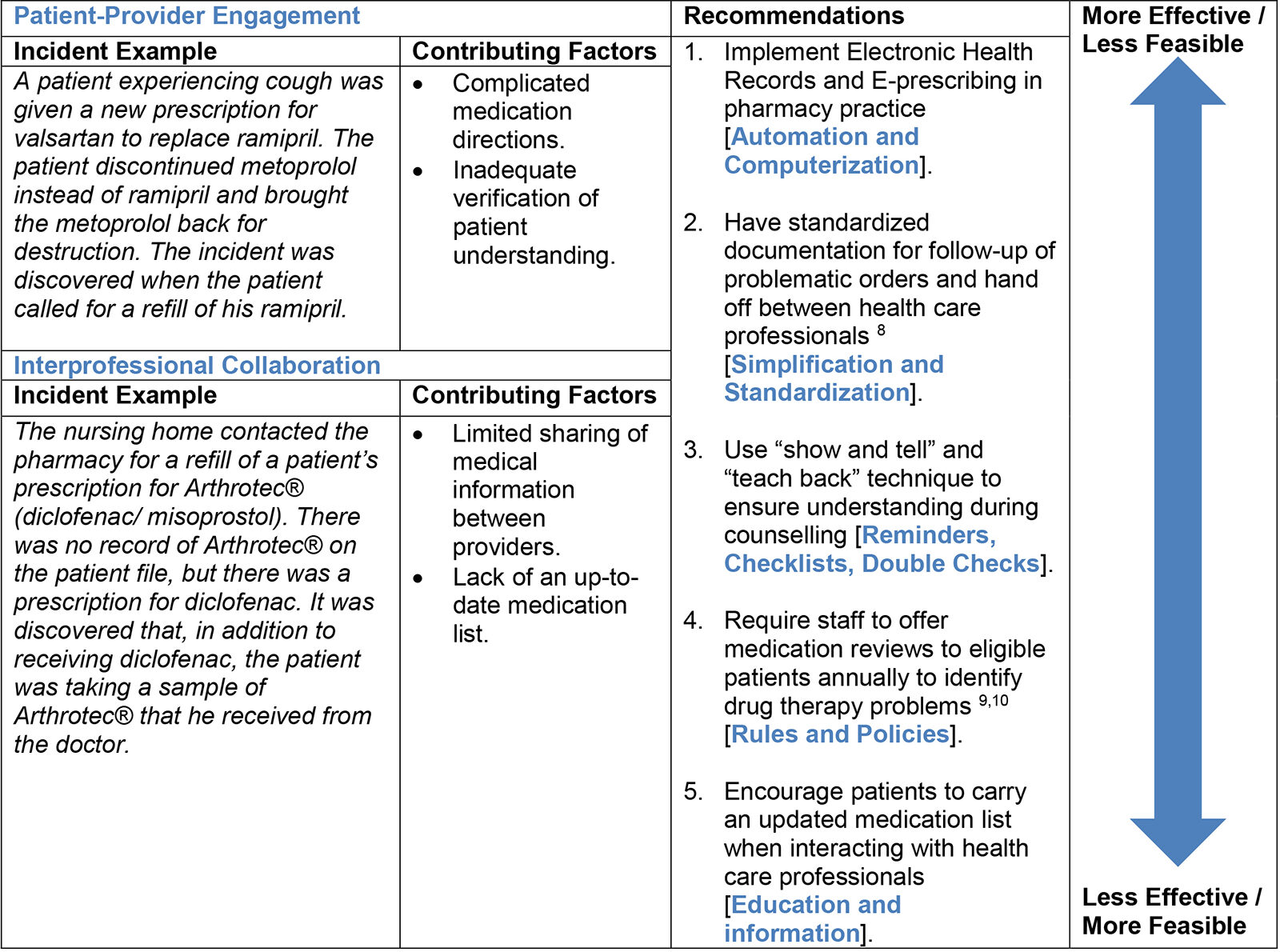
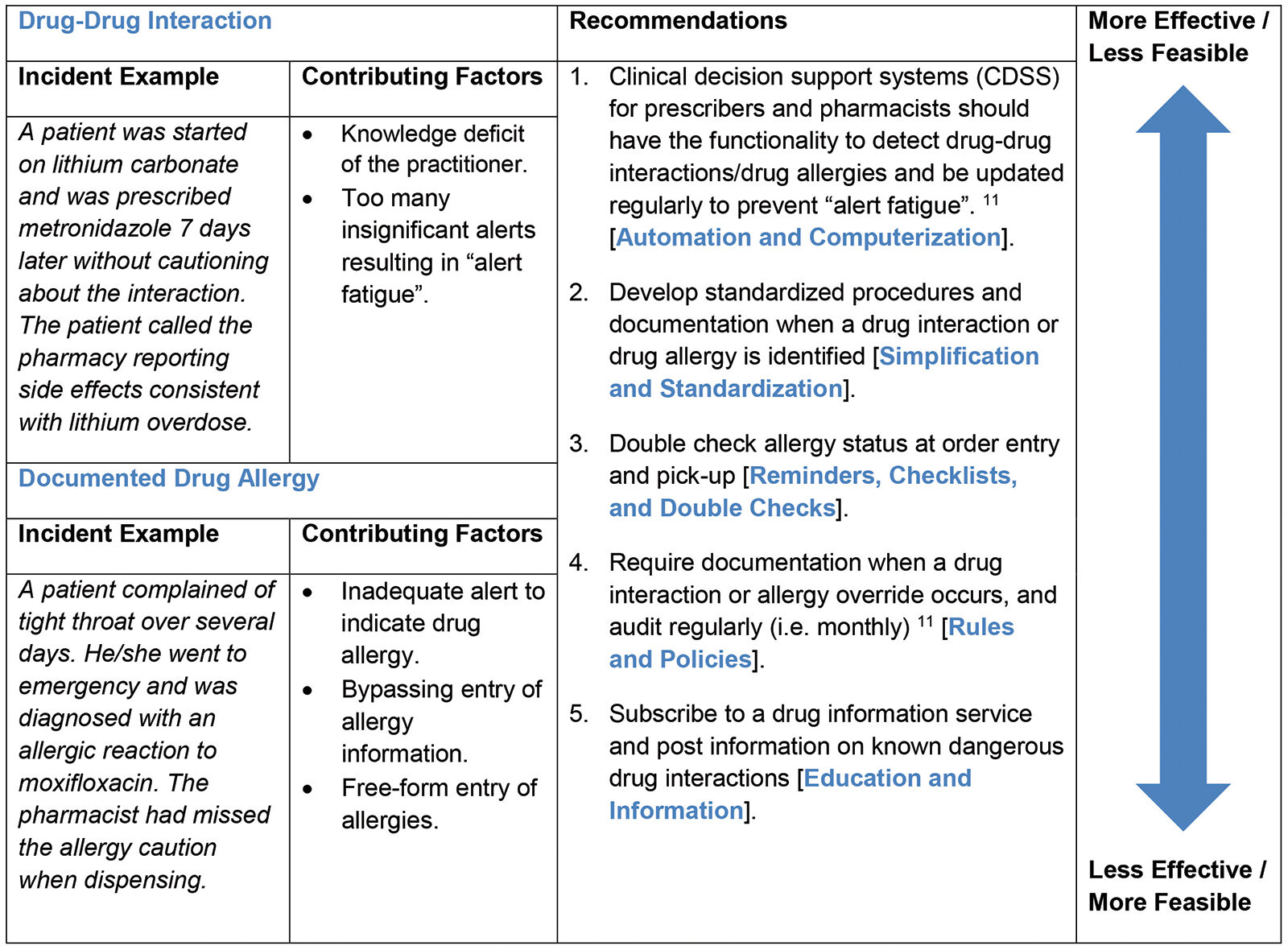
Three main themes were identified: (1) High Risk Processes in the Pharmacy; (2) Communication Gaps; and (3) Preventable Adverse Drug Reactions (Table 1). Subsequent sub-themes were then derived from these three main themes accordingly (Table 1). Incident examples, contributing factors and recommendations based on the hierarchy of effectiveness for CQI solution development (Figure 1) are also provided below (Tables 2, 3, and 4).
Table 1: Main Themes and Subthemes Derived from the Multi-Incident Analysis of Medication Incidents Associated with Patient Harm
Figure 1: Designing Effective Recommendations Using the Hierarchy of Effectiveness
Table 2: Theme 1 – High Risk Processes in the Pharmacy
Table 3: Theme 2 Communication Gaps
Table 4: Theme 3 Preventable Adverse Drug Reactions
CONCLUSION
Medication incidents associated with patient harm present an opportunity for learning and improvement of the medication-use system in community pharmacy. This multi-incident analysis revealed that high risk processes, communication gaps, and preventable adverse drug reactions were the most common themes for reported medication incidents associated with patient harm.
When designing safety solutions using the hierarchy of effectiveness (Figure 1), we have provided different recommendations that can be implemented in your practice based on feasibility and effectiveness. In particular, implementing independent double checks is a feasible strategy for preventing incidents associated with high-risk processes. Furthermore, developing standardized communication and documentation is necessary to ensure safe and effective medication use within the circle of care. Finally, improving the effectiveness of clinical decision support systems utilized by health care practitioners will help mitigate the potential for preventable adverse drug reactions. We hope our findings from this multi-incident analysis help improve medication safety by providing a platform for reflection and shared learning.
ACKNOWLEDGEMENT
ISMP Canada would like to acknowledge support from the Ontario Ministry of Health and Long-Term Care for the development of the Community Pharmacy Incident Reporting (CPhIR) Program (http://www.cphir.ca). The CPhIR Program contributes to the Canadian Medication Incident Reporting and Prevention System (CMIRPS) (https://www.ismp-canada.org/cmirps/index.htm). A goal of CMIRPS is to analyze medication incident reports and develop recommendations for enhancing medication safety in all healthcare settings. The incidents anonymously reported by community pharmacy practitioners to CPhIR were extremely helpful in the preparation of this article.
REFERENCES
- Definition of Terms. Institute for Safe Medication Practices Canada. Available from: https://www.ismp-canada.org/definitions.htm
- Medication without harm – Global patient safety challenge on medication safety. Geneva: World Health Organization, 2017. License: CC BY-NC-SA 3.0 IGO. Available from: http://apps.who.int/iris/bitstream/10665/255263/1/WHO-HIS-SDS-2017.6-eng.pdf?ua=1&ua=1
- Paquette A. Special report: Top drugs of 2015. Pharmacy Practice Plus 2016. Available from: http://www.canadianhealthcarenetwork.ca/
- Hutchinson A, Young TA, Cooper KL, McIntosh A, Karnon JD, Scobie S, et al. Trends in healthcare incident reporting and relationship to safety and quality data in acute hospitals: results from the National Reporting and Learning System. Qual Saf Health Care 2009 Feb 1;18(1):5-10.
- Tsang J, Ho C. Complexity and Vulnerability of Compliance Pack Preparation. Pharmacy Connection 2014 Winter;32-37.
- Elliot RA. Appropriate use of dose administration aids. Aust Prescr 2014;37:4650.
- Chung N, Ho C. Lessons Learned from a Provincial Pilot Study. Pharmacy Connection 2016 Winter;34-39.
- ISMP Canada. Look-alike/sound-alike drug names: Can we do better in Canada? ISMP Canada Safety Bulletin 2004;4(2):1-2.
- ISMP Canada. Errors Associated with Hospital Discharge Prescriptions: A Multi-Incident Analysis. ISMP Canada Safety Bulletin 2017;17(1):1-7.
- ISMP Canada. 5 Questions to Ask About Your Medications. SafeMedicationUse.ca Newsletter 2016;7(7):1-2.
- ISMP Canada. Aggregate Analysis of Medication Incidents Involving Drug Interactions. ISMP Canada Safety Bulletin 2012;12(5):1-4.