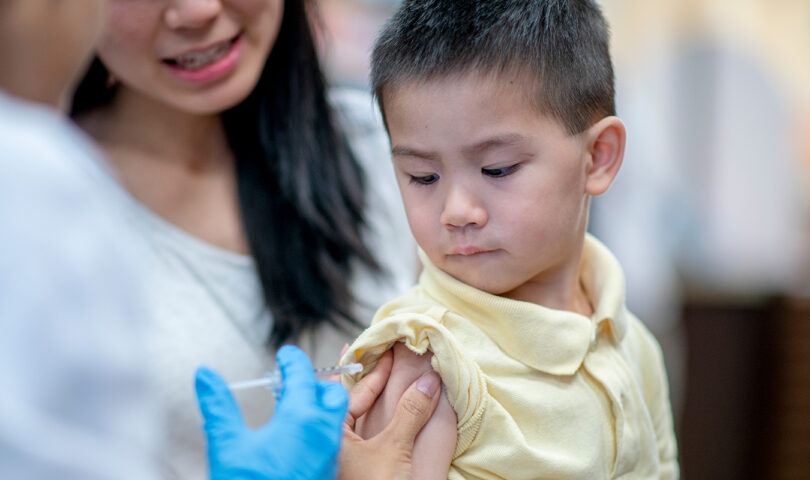
Pharmacy professionals have an important role to play in helping meet the needs of patients while contributing to healthier communities and a high performing, collaborative health system. It is expected that their role will become even more significant with anticipated legislative changes that will expand their scope of practice – some of which could soon be approved by the provincial government.
In May 2019, the Minister of Health asked the College to submit draft regulations that would enable pharmacists to administer the flu vaccine to children as young as two years old, renew prescriptions in quantities of up to one year’s supply, and administer certain substances by injection and/or inhalation for purposes that are in addition to patient education and demonstration.
These draft regulations were submitted to the Minister and Ministry of Health in November 2019 following a collaborative, system-wide engagement process that involved patients, registrants, physicians and other healthcare providers, professional associations, regulators and others. Their insights shaped the development of regulatory amendments, and are informing the work required to support expanded scope of practice for registrants.
Over the summer, the government posted the draft regulations on the Ontario Regulatory Registry for a 45-day public consultation. No changes will come into effect until the government has approved the regulatory amendments.
MINOR AILMENTS
In June 2020, the College also submitted draft regulations to the Minister of Health that would allow pharmacists to prescribe for certain minor ailments, including:
- Urinary tract infections
- Dermatitis
- Insect bites, including tick bites, as well as hives
- Conjunctivitis
- Allergic rhinitis
- Candidal stomatitis
- Herpes labialis
- Hemorrhoids
- Gastroesophageal Reflux Disease
- Dysmenorrhea
- Musculoskeletal sprains and strains
- Impetigo
The minor ailments draft regulations were developed after several months of consultation with the Minor Ailments Advisory Group as well as feedback from registrants, the public, patient advisors, professional associations, health regulators and other stakeholders.
PROVIDING SUPPORT FOR PHARMACY PROFESSIONALS
While the government must approve the relevant regulations before pharmacists can practice these expanded scope activities, the College has been developing resources to support the profession in implementing the changes safely and with confidence.
For example, to ensure pharmacists fully understand their ethical, legal and professional obligations of prescribing for minor ailments, the College’s Board of Directors has approved the requirement for all Part A pharmacists to complete mandatory orientation on the regulatory requirements and practice expectations that is free to registrants and not to exceed two hours in length. It is important to note that the mandatory orientation requirement does not relate to clinical training; the College will continue to expect that pharmacists who engage in this new authority, once approved by government, will have the required knowledge, skills and judgement to do so safely and ethically and in accordance with the standards of practice, Code of Ethics, and current clinical resources.
Once the government approves regulation changes, the College will share relevant tools and resources to support expanded scope activities. Further details about the ways the College will be supporting pharmacy professionals will be shared in future editions of e-Connect and on the College website.