The National Association of Pharmacy Regulatory Authorities (NAPRA) has released new standards on non-sterile compounding. College Council adopted the Model Standards for Pharmacy Compounding of Non-Sterile Preparations at their meeting in December 2017. The Model standards are accompanied by a Guidance Document for Pharmacy Compounding of Non-Sterile Preparations.
WHY ARE THESE STANDARDS IMPORTANT?
These standards are an important way of protecting patients and increasing patient safety.
The aim of the standards is to provide pharmacists and pharmacy technicians who compound non-sterile preparations with the standards necessary to evaluate their practice, develop service-related procedures and implement appropriate quality controls for both patients and compounding personnel.
Model standards represent the minimum requirements that must be met regardless of practice site and against which performance can be measured. The College gave pharmacy professionals the opportunity to weigh in on the draft standards through a consultation in late 2016. NAPRA also incorporated input from pharmacy regulatory bodies and experts across the country. It is the intention of the College that, whenever possible, national standards will be adopted.
WHO DO THESE STANDARDS APPLY TO?
The standards apply to ALL pharmacies that perform any type of non-sterile compounding in any quantity (whether once in a while or every day). Not sure if you are compounding? Read the recent Pharmacy Connection article Compounding: Are You Doing It?
WHAT DO THESE STANDARDS REPLACE?
These standards effectively replace the College’s 2006 Guideline for Compounding Preparations and NAPRA’s Guidelines for Pharmacy Compounding (2006).
WHAT IS THE DEADLINE FOR THE IMPLEMENTATION OF THE STANDARDS?
The College is currently working towards establishing an appropriate implementation deadline. However, pharmacies are expected to begin implementation of the standards now.
HOW CAN YOU BEGIN IMPLEMENTATION?
Pharmacy professionals should be proactive:
- Review and become familiar with the standards and guidance.
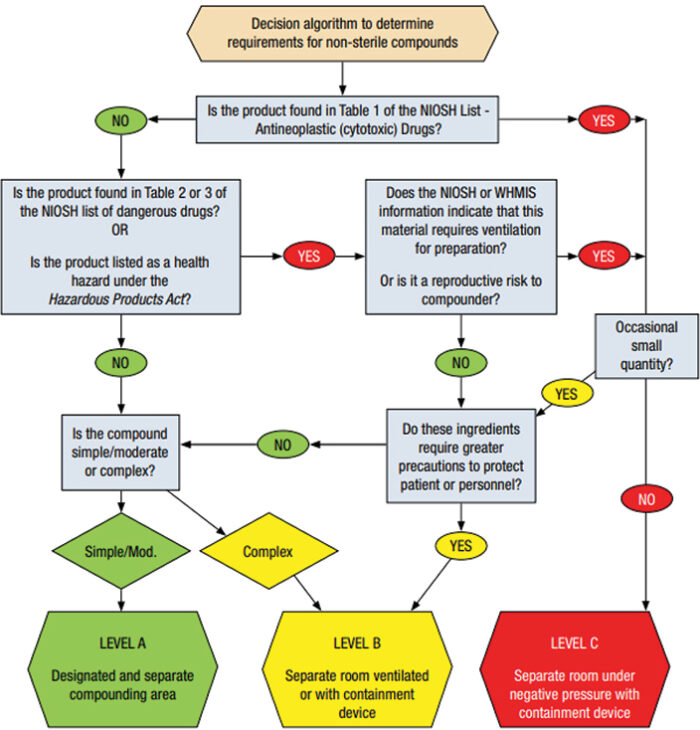
- Undertake a risk assessment to identify the appropriate level of requirements to minimize contamination of each compounding product and to provide adequate protection for personnel. Use Diagram 1: Decision algorithm for risk assessment found in the guidance document (and below).
- Conduct an assessment to identify gaps between the standards required in the pharmacy and your current practice, processes and compounding environment. The guidance document can then provide more details on how the standards can be achieved.
- Start work to close those gaps. For example, pharmacies can begin by reviewing current policies and procedures, master formulations and Safety Data Sheets. Pharmacies and pharmacy professionals can start looking for resources (e.g. education) that are relevant to any gaps in their knowledge or processes.
It is the expectation of the College that this work should begin now; there is no need to wait until the implementation deadline is announced.
Diagram 1: Decision algorithm for risk assessment from the Model Standards for Pharmacy Compounding of Non-Sterile Preparations Guidance Document.
Related stories
Compounding: Are You Doing It?