While there is no single way to detect a forgery, there are several red flags or other factors that should prompt you to take additional care in verifying that the prescription is legitimate. Ultimately, it is the responsibility of pharmacists to ensure that all prescriptions received are complete, authentic and appropriate.
In addition to receiving reports about the loss or theft of controlled substances, Health Canada also has teams of inspectors who, using a risk-based approach, assess pharmacies for compliance with the Controlled Drug and Substances Act (CDSA) and its regulations.
Here are some of the top tips from Health Canada’s Controlled Substances Program:
1. Critically Assess the Prescription
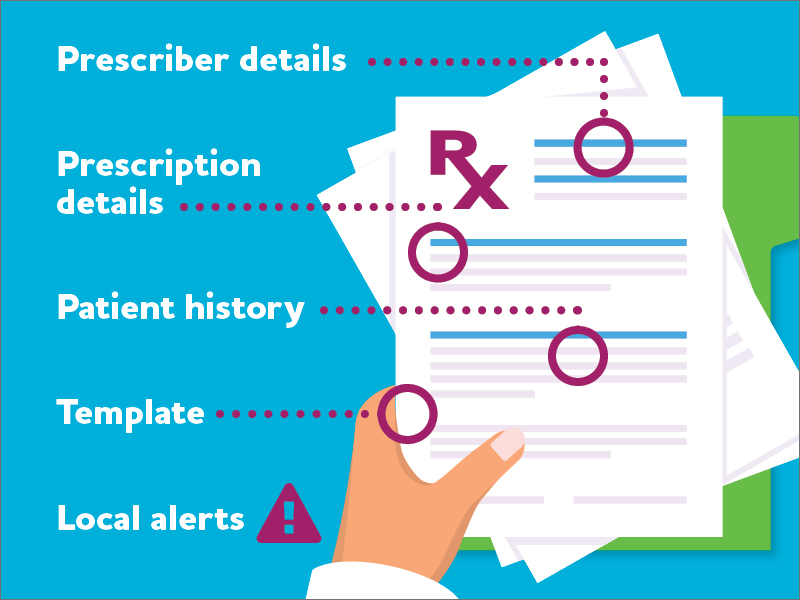
Prescriber details
- Play close attention to out of province prescriptions.
- Generally, the prescribers’ office and/or the patient should be a reasonable distance from the pharmacy.
- Check the prescriber’s public register entry for any restrictions on prescribing.
Prescription details
- Ensure the quantity and instructions for use make sense for the indication and align with best practices for controlled substances.
- Be alert for spelling errors.
- Check for and verify with the practitioner any quantities or directions that are struck out or if amounts look like they have been adjusted.
Template
- With electronically generated prescriptions, check for irregular changes in font styles or sizes and page layout issues that differ from standardized layouts.
- Look for any evidence of photocopying.
Patient history
- Be aware of patients who consistently come in requesting early refills.
- Take extra care when multiple patients are at the same physical address or if the patient presents prescriptions from multiple prescribers.
- Always verify with the practitioner if a patient comes in requesting refills of a prescription recently picked up and claiming it was lost or stolen.
Local alerts
- Be aware of alerts from surrounding pharmacies and/or prescribers and stay connected with your local police services.
- The Narcotics Monitoring System (NMS) alerts pharmacies of potential issues such as patients who obtain prescriptions for a monitored drug from more than one prescriber or from more than one pharmacy. NMS also alerts pharmacies when a prescriber has notified the Ministry of Health about a previously identified forged prescription for a monitored drug and/or stolen prescription pad.
2. Remain Alert to Circumstances Around the Prescription Drop Off
- Be aware of those coming in close to closing time/late at night.
- Be aware of those conveying urgency and unwillingness to wait for verification.
- Do not feel pressured to dispense if you have not been able to verify the prescription and the patient or an agent on their behalf shows up early the next day demanding their prescription.
- Do your due diligence/verification when agents on behalf of the patient are dropping off and/or picking up prescriptions.
3. Use Best Practices for Verifying Prescriptions

Do not automatically trust the information provided on the prescription about the prescriber.
When contacting the practitioner to verify the prescription, you should look up their contact information where possible instead of relying on what was provided on the prescription. Always check the doctor’s name and/or registration number against the provincial college listing.

Take extra care with new prescriptions and any change in dosage.
When dealing with new patients and prescribers, always verify contact information and identification. Re-verify any changes in dosage and quantity with the prescriber.

Establish appropriate pharmacy procedures and ensure staff are trained on them.
Do not forget to educate all staff, including non-regulated and relief staff, on your procedures for verifying and processing prescriptions. When a forgery is identified, ensure this is flagged in your system for any future prescriptions you may receive from the patient or prescriber and shared with the pharmacy team for learning.

Be part of a network.
Share knowledge of fraudulent scripts with local pharmacies in your community.
Reporting Responsibilities
- All filled forgeries of controlled substances must be reported to Health Canada’s Office of Controlled Substances within 10 days of its discovery. Reports can be made through the Health Canada E-Services Portal.
- Registrants should review the College’s Forgery: Management and Reporting of Fraudulent Prescriptions fact sheet for more information on how to handle a forgery and when to report to police.
- Forgeries should NOT be reported to the Ontario College of Pharmacists.













