Health Canada is calling on healthcare professionals, including pharmacists and pharmacy technicians, to support a program focused on ending the illegal advertising of drugs and medical devices.
The program—Stop Illegal Marketing of Drugs and Devices—was created to help increase public protection through education, engagement and reporting tools. The program aims to:
- create an understanding of legal and illegal practices;
- provide a quick and easy tool to file a complaint to Health Canada; and
- keep healthcare professionals from inadvertently perpetuating illegal advertising when discussing health products and/or services with patients.
Health product advertising
Health product advertising is the direct or indirect promotion surrounding the sale or use of any drug or device conducted by any means including print, Internet and broadcast. Only drugs and devices that have been authorized for sale by Health Canada may be advertised in Canada.
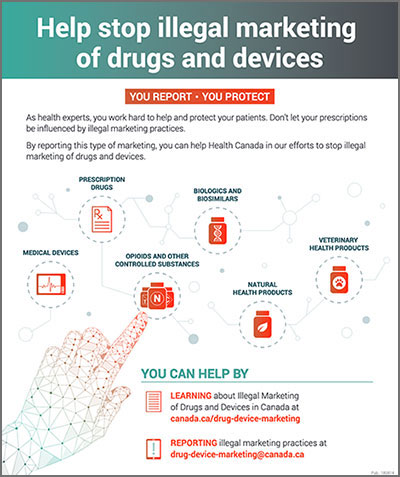
Areas under renewed marketing scrutiny include prescription drugs, biologics and biosimilars, opioids and other controlled substances, medical devices, veterinary health products and natural health products. In cases where the illegal marketing of drugs and medical devices is identified, Health Canada is taking action as required, including recommending criminal charges where appropriate. The complaint process is available on Health Canada’s website.
- are false, deceptive or misleading;
- do not provide a balanced representation of benefits and risks; and
- are not consistent with the terms of market authorization of the product.
As marketing materials provided by the pharmaceutical industry are among the information sources used by healthcare professionals to make decisions about patient care, Health Canada is striving to ensure they accurately convey the benefits and risks of products.
How to confirm product status and verify the information provided to you
To confirm whether the indications of a drug or device that is being marketed has been authorized by Health Canada, check for proof of preclearance by an advertising preclearance agency (APA) recognized by Health Canada (APA logo).
If proof of preclearance is not available, consult the databases for the product lines:
- medical devices, the Medical Devices Active Licence Listing
- therapeutic, biologic and veterinary drugs, the Drug Product Database
- natural health products and homeopathic medicines, the Licensed Natural Health Products Database
- veterinary health products, the List of Notified Products