By: Heather Kidston, RPh, FSVHP
How many times per week are you presented with a prescription for an animal? Or asked questions about purchasing an OTC product for a pet? Can you name two reliable veterinary drug references?
During my 13 years as a community pharmacist, I was frequently asked questions about drug therapy for clients’ pets, and filled many prescriptions for animals. As a veterinary hospital pharmacist, I now recognize how often I incorrectly assumed that I could safely extrapolate my “human” pharmacy knowledge to other species.
As per the Drug and Pharmacies Regulation Act, a drug is defined as any substance that is used in the diagnosis, treatment, mitigation or prevention of a disease in humans, animals or fowl. The intent of this article is to increase awareness of some of the challenges that pharmacists face as a result of animals being included in this definition.
SCENARIO #1
Mrs KL approaches pharmacist MP on his Saturday evening shift. She explains that she has run out of her syringes to administer insulin to her beagle, Beetle. MP has often dispensed prescriptions from the local vet for both insulin and syringes. Based on Beetle’s 5 unit BID dose, MP provides Mrs KL with BD 3/10cc syringes.
How could this interaction put Beetle’s health at risk?
Pharmacists are accustomed to insulin being 100 U/mL. Many veterinary patients are prescribed human insulin. Others, like Beetle, use veterinary-only Caninsulin (porcine insulin zinc), which is 40 U/mL. Caninsulin syringes are calibrated to 40 U/mL. When Mrs KL draws Caninsulin to the “5 units” mark on the BD syringe, Beetle will receive only 40% of the correct insulin dose.
SCENARIO #2:
Client JD presents a prescription for his dog, Spot, for Clavamox 250mg tablets. He requested a prescription from his veterinarian because he heard that it was less expensive at a community pharmacy. The pharmacist, AB, investigates, and finds that, as the brand name suggests, Clavamox is a veterinary drug containing amoxicillin and clavulanic acid. She informs JD that she doesn’t carry the brand that the vet has prescribed, but will substitute the human generic amoxicillin-clavulanic acid 250 mg tablet.
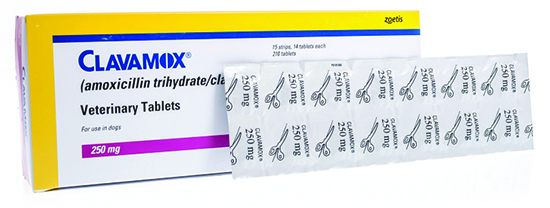
How could this interaction put Spot’s health at risk?
Veterinary labelled amoxicillin-clavulanic acid tablets contain the two ingredients in different ratios than human-approved products. Clavamox 250mg contains 200-50 (250mg refers to the total of BOTH compounds), while human generics contain 250-125 (250mg refers to amoxicillin content only). Spot was dispensed 50mg more amoxicillin and 75 mg more clavulanic acid per dose than he was prescribed. Although 50 mg of extra amoxicillin may not be clinically relevant, the higher dose of clavulanic acid places Spot at higher risk of GI side effects.
In addition to veterinary labelled drugs, species-specific pharmacokinetics and toxicities complicate the provision of care to animals. These will be discussed in a future edition of Pharmacy Connection.
So where does one find veterinary-specific drug information? A great resource to have on hand is Plumb’s Veterinary Drug Handbook. Written and reviewed by pharmacists, Plumb’s reads just like the CPS. The Health Canada drug database also provides information on both human and veterinary drugs. For those who wish to learn more, online veterinary pharmacy continuing education courses are offered at some American pharmacy schools.
It is a pharmacist’s professional responsibility to acknowledge when he or she lacks sufficient knowledge to provide a service. The challenge is recognizing those knowledge deficits. Failing to properly consider drugs marketed for veterinary use, pharmacokinetic differences and toxicities could result in inadvertent harm to the animal patient.
Pharmacists and veterinarians share a collective duty of care. By working together, we can address these challenges and form an alliance that places animal (patient) safety first.
Related stories
Health Canada Introduces New Rules for Pharmacies that Compounding APIs for Veterinary Use













