If I have a room for non-sterile compounding of hazardous drugs, can I also make non-hazardous compounds in it?
The NAPRA Guidance Document For Pharmacy Compounding Of Non-Sterile Preparations explains that compounding of hazardous non-sterile preparations should occur in a separate room specifically dedicated for this purpose. Also, it is strongly recommended that dedicated equipment is used to compound hazardous preparations. Another option is to use disposable equipment where possible to reduce the chances of cross-contamination.
The level of requirements needed (i.e., B or C) depends more on the risk(s) posed by the hazardous product than on the complexity of the preparations. Drugs listed in the NIOSH† Group 1 (antineoplastic/cytotoxic) require handling in the greater precautions provided by Level C (e.g., a closed-off room under negative pressure, filtered air exhausted to the outside, etc.) to avoid contaminating the environment and to further protect personnel. Similarly, compounding with drugs categorized by WHMIS‡ as a health hazard that are very irritating to the respiratory tract, skin and/or mucous membranes should also take place in a Level C room.
If it is not possible to have a dedicated room for hazardous compounding, at an absolute minimum there should be assurances that the area is meticulously cleaned before non-hazardous preparations are compounded. To prevent any risk of cross-contamination with the hazardous materials, the compounding area, equipment and accessories must be deactivated, decontaminated and cleaned as described in Section 9, immediately after compounding. For occasional compounding of non-sterile hazardous products, a C-PEC used for sterile hazardous compounding (e.g., Class II BSC or CACI) may be used, provided it is decontaminated, cleaned and disinfected before compounding the non-sterile product and again before resuming sterile compounding in that C-PEC. Policies and procedures for cleaning must be implemented, and personnel must be trained and assessed to ensure they are qualified to perform these tasks.
†National Institute for Occupational Safety and Health
‡Workplace Hazardous Materials Information System
Section 9 in the NAPRA Guidance Document states that “the compounding of hazardous nonsterile preparations requires safety measures to protect personnel and the environment” and refers to the NIOSH “hierarchy of controls” diagram depicting various levels of controls that can be implemented. Can you explain what this means?
The NIOSH website explains that a hierarchy of controls is “a means of determining how to implement feasible and effective control solutions” to minimize the risk of exposure to occupational hazards. Compounding supervisors should review the descriptions of each level of control. The diagram below (from the November 4th OCP webinar) provides examples of what the various controls might be in the context of pharmacy compounding.
The Guidance Document states that if there are “extra steps that must be taken to mitigate the risks” associated with compounding a particular preparation, these must be documented on the risk assessment along with “references confirming that these steps actually will minimize risks to the quality of the product and safety of personnel.” It is the responsibility of the compounding supervisor and manager to evaluate and choose the appropriate ‘controls’ depending on the type of risk posed by a hazardous product.
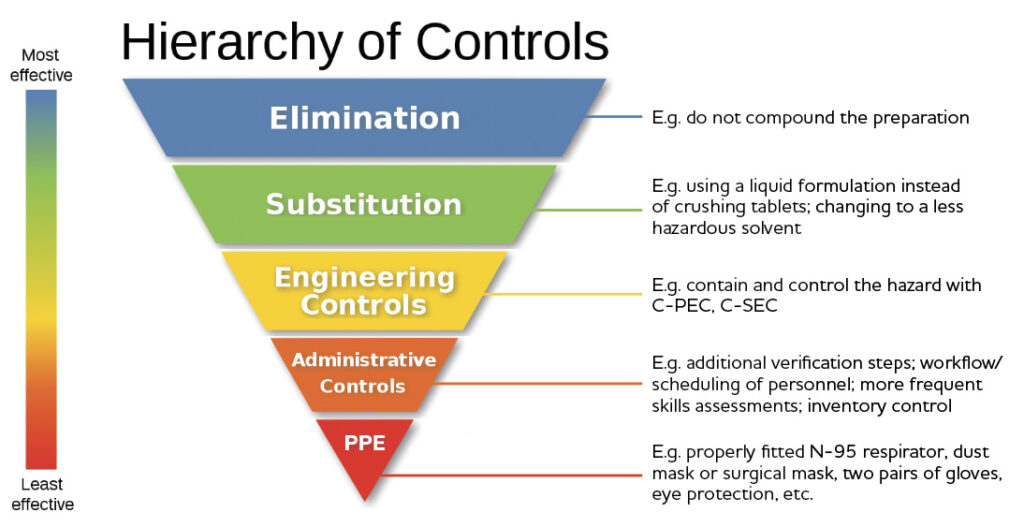
Adapted from: https://www.cdc.gov/niosh/topics/hierarchy/default.html
ADDITIONAL RESOURCES
- November 4th webinar and slides
- Pharmacy Connection Article:
- Frequently Asked Questions:
- As part of our risk assessment, should we follow the WHMIS list or the NIOSH list? I don’t understand the difference between the two
EXTERNAL RESOURCES
- Best Practices for the Safe Handling of Hazardous Drugs (WorkSafe BC)
- Prevention Guide – Safe Handling Of Hazardous Drugs (ASSTSAS QC)
- ASHP Guidelines on Handling Hazardous Drugs